As lithium iron phosphate (LFP) batteries continue to gain prominence across mobility and stationary storage applications, the emphasis on optimizing cathode performance has extended beyond chemical formulation to the physical attributes of the active materials. Among these, particle size, morphology, surface area, and structural uniformity—both at the precursor and final cathode stage—have emerged as defining factors influencing electrochemical behavior, cell stability, and manufacturing efficiency.
This article presents a consolidated view on how particle engineering, beginning with iron phosphate (FePO₄) and continuing through the LFP synthesis and electrode fabrication process, plays a critical role in determining performance outcomes and production scalability.
Role of Iron Phosphate Particle Engineering in LFP Synthesis
Iron phosphate, as the key precursor for LFP synthesis, sets the foundation for the structural and functional characteristics of the final cathode active material. Uniform particle size distribution and controlled morphology ensure homogeneous lithium integration during solid-state synthesis, reduce phase inhomogeneities, and enable efficient formation of the olivine-type crystal structure characteristic of high-quality LFP.
- Stoichiometric control and homogeneity: Narrow particle size distributions contribute to improved reaction uniformity and compositional balance during calcination.
- Surface structure and shape retention: The morphology of FePO₄ can influence the preservation of desirable physical traits in the final LFP product, particularly under tightly regulated synthesis parameters.
A precursor with appropriate surface area, low agglomeration, and high flowability contributes significantly to the reproducibility and efficiency of large-scale LFP synthesis.
Impact of Morphology and Particle Size on LFP Cathode Performance
Once synthesized, LFP as a cathode active material must exhibit a balance of electrochemical activity, mechanical robustness, and process compatibility. The microstructure of the LFP particles — inherited in part from the precursor — is essential to achieving this balance.
1. Lithium-ion Transport and Kinetics
Smaller particle sizes reduce the diffusion path for lithium ions, enhancing rate capability. However, overly fine particles (<200 nm) can lead to excessive surface area, increasing side reactions and complicating electrode slurry formulation.
2. Tap Density and Electrode Loading
Tap density directly influences the volumetric energy density of the electrode. Optimized morphology — particularly spherical or ellipsoidal particles with a narrow PSD — facilitates efficient packing, reducing void space and enabling higher active material loading per unit volume.
3. Surface Area and Carbon Coating Uniformity
Given the inherently low electronic conductivity of LFP, uniform carbon coating is essential. A consistent and defect-free particle surface allows for homogeneous carbon distribution during post-synthesis treatment, enhancing both electron transport and cycle life.
4. Mechanical Stability and Cycling Durability
Well-structured LFP particles resist degradation during lithium insertion/extraction cycles. Morphological features such as minimal porosity and structural symmetry contribute to improved mechanical integrity under repeated cycling and elevated operating conditions.
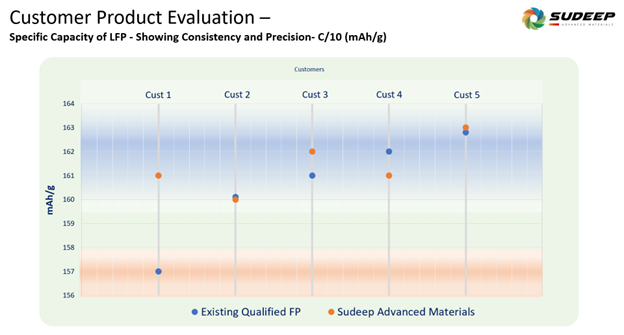
Sudeep Advanced Materials: Focused Approach to Particle-Level Precision
At Sudeep Advanced Materials, our production of iron phosphate precursors is based on a systematic approach to particle engineering, combining precipitation chemistry, advanced drying technologies, and post-processing control. Our process capabilities enable:
- Customizable particle size distribution (D50: <8 µm) with a narrow span
- Spherical, free-flowing particles with minimal agglomeration
- Controlled surface area (BET: 5–10 m²/g) to ensure electrolyte interaction without excessive reactivity
These characteristics support consistent synthesis of high-quality LFP and downstream compatibility with various electrode manufacturing processes. Our infrastructure is designed to support customization at industrial scale, ensuring alignment with specific performance and processing requirements of battery and cell manufacturers.
Conclusion
The performance of LFP batteries is inherently linked not only to their chemical composition but also to the physical attributes of both precursor and final cathode materials. Particle size distribution, morphology, and surface characteristics have demonstrable impacts on rate capability, energy density, mechanical durability, and manufacturing efficiency.
As battery technologies evolve to meet the demands of next-generation electric mobility and grid-scale storage, material design strategies must extend beyond chemistry to include microstructural engineering. At Sudeep Advanced Materials, we are committed to advancing this frontier through the development of tailored iron phosphate materials and scalable particle engineering solutions — enabling the next phase of high-performance, sustainable battery technologies.